Drug firm Jubilant Pharmova Ltd on Friday (June 7) said the United States Food and Drug Administration (FDA) has issued three observations for its contract manufacturing facility operated by Jubilant HollisterStier LLC in Spokane, Washington.
"The USFDA has issued 3 observations pursuant to the completion of the audit. The company will submit an action plan on the observations," Jubilant Pharmova said in a regulatory filing.
Also Read: Wardwizard Innovations bags $1.29-billion EV order from Philippines company
"The United States Food and Drug Administration (USFDA) has concluded an eight-day audit of the Jubilant HollisterStier LLC’s contract manufacturing facility located in Spokane, Washington (USA)," the company said.
Jubilant HollisterStier LLC is a subsidiary of Jubilant Pharma Holding Inc, which is a subsidiary of Jubilant Pharma Ltd, Singapore, a wholly-owned subsidiary of the company.
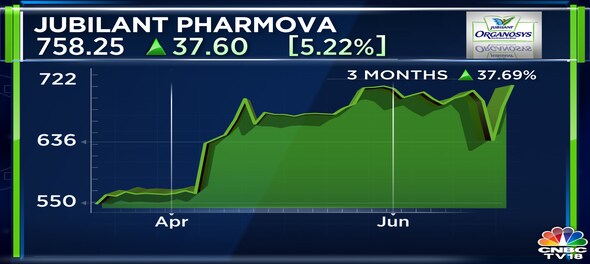
Shares of Jubilant Pharmova Ltd ended at ₹758.25, up by ₹37.60, or 5.22%, on the BSE.
Also Read: Dr Reddy's manufacturing unit in Andhra Pradesh gets 4 observations from US FDA
